|
-
Screening
and finding best candidate molecules for generic launch, for your territory
of interest in any therapeutic area
-
Crystal
clear patent situations &, launch possibilities in each country
-
Pin-point
Dossier selection for licensing based on our continuous
literature and screening program (see
some samples)
-
Ready
Dossiers with proven and completed BE studies that can coupled with
ready, reliable API sources having sufficient and approvable
documentations (having US-DMF, EU- COS, in proper format such in CTD
format)
-
selection
of best partners for Turkish companies from Japan, Europe, USA or elsewhere for
collaboration in all means, such as product exchange, licensing,
technology /know-how exchange, tool manufacturing, etc, for mutual
benefit, win-win situation.
-
Our
company particular focused on the best synergy generating
possibilities between Turkish- Japanese ALLIANCE,
due to several folds, as mentioned in the followings.
-
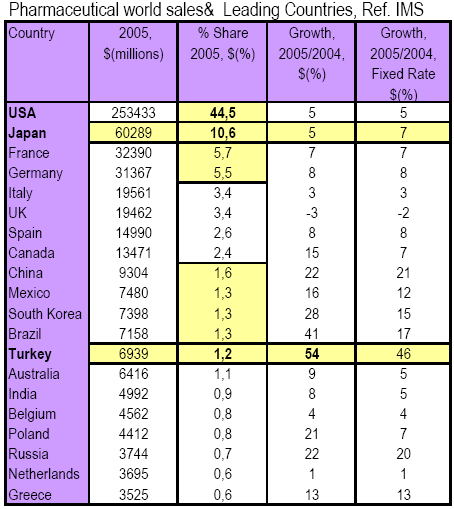
Turkey
is the fastest growing Pharmaceutical market in the world with a 54%
increase from 2004 to 2005, according to IMS data given on the left.
-
Japan
is the 2nd largest
market & largest in Asian market while share
of generics is extremely low as low as 16-17% when compared with those
in the USA, 51%, the UK 52% and Germany 50%.
|


