|
|||||||||||||||||
| API sourcing, selection and evaluation | |||||||||||||||||
We
are very well known by the world's distinct API suppliers. Thus, They very
well know what we need as far as reliability, quality and fastest delivery
times are concerned.  Our expertise extends to all rules, regulations, standards and methodologies that are adopted and followed by international authorities, for instance EMEA and FDA. |
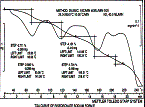
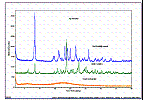
 Our staff are highly educated and experienced in this field of science, including organic synthesis/ chemistry, inorganic chemistry, fermentation, particle and material science as well as in instrumental analysis techniques and have had hands on experience since years on sample characterization, determination and detection.  |
In
one hand, we can establish contacts between very well known suppliers and
the Turkish generic companies and on the other hand, we are seeking new
API suppliers that we can represent them in the fastest growing market of
the world, in Turkey. We are confident that we can make right
selection for your API requirements. If you need any costume made products
for a specific requirement, such as pellets, delayed release forms, OROS
tablets and so on, we can explore best alternatives for you.
Our scope of work covers the followings:
|
|||||||||||||||